A medical device, controversial from its introduction, is the subject of growing concern due to numerous reports of serious and adverse complications arising out of its’ use.
A safety warning has been issued by Health Canada concerning the use of inferior vena cava (IVC) filters, which are implanted into the largest vein in the body to stop blood clots from migrating to the lungs and heart. Canada’s regulatory body announced that more than 120 incidents have been reported as of June 2016.
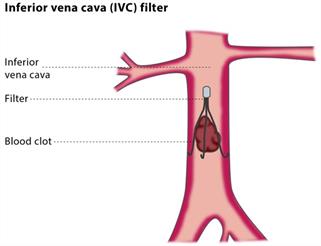
The filters are thin metallic devices which when implanted, expand outwards and the spindles, or legs, fan out which traps blood clots travelling towards the heart and lungs. The complications that have been noted with the filters is that they deteriorate or fracture and can pierce or puncture the vein or heart. In some cases, the breakage of the device and the ensuing splintering of the fine metal piece have led to the death of the patient.
>
As of June 6, Health Canada has received 121 serious incident reports involving the IVC filters. There are 6 companies who manufacture inferior vena cava (IVC) filters, including:
- A.L.N
- Bard Peripheral Vascular
- B. Braun Medical
- Cordis Cashel
- REX Medical, LP
- William Cook, Europe APS And Cook, Inc.
The filters are categorized as “retrievable medical devices”, which means that they can be removed after the device has performed its function. However, it has been determined that due to the breakages or fractures of the filters the thin metal, or portions of it, may migrate, become lost or may become embedded in the venous wall and are no longer removable, even by invasive surgical procedures.
As the issue is becoming more apparent and widespread, one hospital in Toronto has taken a proactive approach and is dealing with the defective filters. The hospital is currently tracking down any patient who underwent the implantation of the device to determine if the filter has degraded, migrated or become lost within the patient’s body.
The same hospital also reviewed data over a 12 year period to make a determination of the scope of the potential problem with its patients. Of note, a study undertaken by the hospital determined that less than 50% of the surgical procedures to remove the IVC device were unsuccessful.
The filters are used regularly in patients who are at an elevated risk of producing clotting and may even be necessary or essential if the patient is unable to take medications or blood thinners to combat blood clots. While there are indeed benefits to the filters, with the recent concerns and reports about the efficacy of IVC filters, it has become apparent that patients who were implanted should attend at their physician to ensure that the device has not failed.

